Pulmonary Metastases – a review
Consultant Paediatric Surgeon,
St Georges Hospital, London
Overview – Pulmonary Metastases (PM)
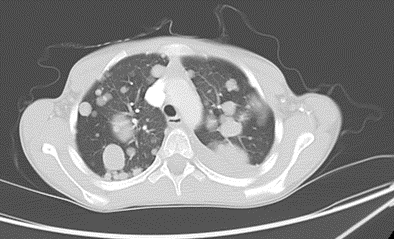
The defining characteristic of a malignant cancer is its ability to spread to a distant site from its origin. In recent years there has been significant improvement made in the survival of children with non-metastatic solid tumours (75-90%). 10-30% of those children with solid tumours however, will be found to have metastases at presentation and these still do not fare as well with an overall survival ranging from 20-70% dependent on the primary tumour type and site.
The most common site for metastases is the lung.
WILMS’ TUMOUR – NEPHROBLASTOMA
- 20% of Wilms’ will have metastases at presentation and of these 95% of these will have pulmonary metastases (PM).
- Estimated 80% survival with PM.
- Nodule(s) of ≥3mm on CT chest are classified as lung metastases.
- Triple neoadjuvant chemotherapy (e.g. doxorubicin) is the primary treatment and metastasectomy should not be thought of as a primary intervention.
- Pulmonary nodules with a complete response on CT after neoadjuvant chemotherapy should be treated according to the local stage of their tumour (For Low and Intermediate Risk).
- For High-Risk patients with evidence of metastases – these should be discussed at National Renal Advisory Group (NRAP)
- After neoadjuvant chemotherapy, biopsy of a representative nodule or resection of residual nodules is recommended if safe and feasible (can be done synchronously with nephrectomy)
- Wilms’ tumour PM respond well to radiotherapy
- Advantages of lung metastastectomy
- Allows histological diagnosis – proof of metastasis, is able to define viable or necrotic malignant tissue
- If complete surgical clearance is obtained for Low and Intermediate Risk disease – and there is no viable or necrotic tumour – this alleviates the need for whole lung radiotherapy (Clinical management guidelines – Renal Tumours CCLG)
- Radiotherapy has been associated with interstitial lung disease and breast cancer on long-term follow up
- If complete surgical clearance of lung metastases can be achieved in High-Risk patients; this potentially reduces the chance of metastatic relapse. However, whole lung radiotherapy is indicated for all High-Risk Wilms’ cases with PM regardless of the response to chemotherapy or completeness of surgical resection.
HEPATOBLASTOMA
- 20% present with pulmonary metastases at diagnosis
- Event free survival (EFS) and overall survival (OS) with metastases has improved recently.
- 3 year EFS and OS: 56%, 62% respectively (SIOPEL-3HR study)
- Pulmonary lesions will be considered malignant if a solitary nodule is >10mm or there are several nodules with at least one > 5mm.
- If there is doubt, then a surgical biopsy should be discussed
- Cisplatin-based neoadjuvant chemotherapy regimes will achieve metastatic resolution in the majority of cases (>50%)
- SIOPEL – 4 – increased dose of cisplatin with improved metastatic response
- Pulmonary metastastectomy remains the only curative option in conjuction with primary hepatic resection or liver transplantation for those patients with a partial response or residual nodules after chemotherapy.
- Still under debate as to timing of lung resection – before, after or simultaneous to the primary tumour.
- Contraindications to pulmonary surgery include
- Inability to achieve a complete resection without preserving adequate lung
- Uncontrolled primary tumour
OSTEOSARCOMA
- 20% of children will present with a PM at presentation and another 20% eventually develop a metachronous PM.
- The overall survial in patients with metastatic disease is only 10-30%
- Osteosarcoma responds poorly to both chemotherapy and radiotherapy
- Complete resection of all PM along with local disease is vital to improve the chance of long term survival.
- Survival benefit has even been shown after repeated metastactectomies.
- Spiral CT with 1mm slices is more sensitive at picking up metastases
- Contraindications to pulmonary metastasectomy include:
- Unresectable disease including metastases to other sites or rapidly progressing disease
- Miliary disease, hilar node or chest wall involvement are relative contraindications
THORACOTOMY
- Historically this has been the technique of choice especially when there are multiple metasases. A thoracotomy approach was favoured in order to palpate the lung and detect nodules too small (micrometases) to be identified on standard radiological examinations (i.e. typically <5mm in diameter). Tumours such as osteosarcoma also contain osteoid which can easily be palpated manually further aiding complete macroscopic resection of all metastases. The disadvantages include pain, increased length of hospital stay, inability to be able to palpate all metastases, chest wall deformities and may make further surgery more challenging.
THORACOSCOPY
- CT scanning has now improved with the use of thin-slice helical CT-scans and the clinical relevance of micrometases (<3mm) is not known. Solitary or oligometastatic (2-5) pulmonary disease (particularly if only present in one hemithorax) can now be treated with thoracoscopy without the need for formal bimanual examination. Minimally invasive sealant devices such as staplers and ligasure have further aided VATS (video assisted thoracoscopic surgery).
LOCALISATION TECHNIQUES
These techniques can act as surgical adjuncts and help localise PM allowing for more parenchymal sparing surgery and accurate localisation of deep, non palpable or barely visible lung metasases.
Hook wire
A hook wire can be used to localise superficial and deep pulmonary nodules prior to VATS surgery. The hook-wire is placed into the lung parenchyma. The tip of the hook wire is left as close to or through the nodules as possible. Complications include wire dislodgment, pneumothorax or parenchymal bleeding.
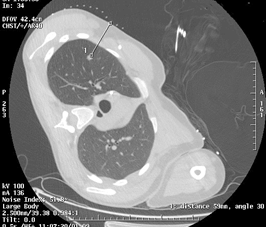
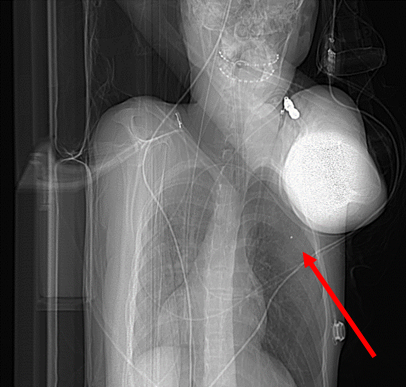
Microcoil
Hook wires have been the most widely used for CT guided localisation of PM prior to video assisted thoracic surgery (VATS). However microcoils have started replacing them as the preferred technique. Microcoils are less likely to get displaced, result in smaller resected surgical specimens and reduced patient pain (see figures 2-5).
 |
 |
 |
 |
Tattoo
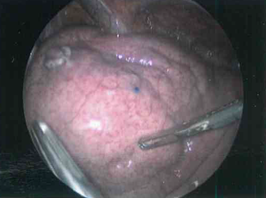
Methylene blue (Fig. 3) has been used to mark out lung metastases similarly to that used during colonoscopy for colonic cancers. It is ideally injected as a subpleural bleb otherwise it can leak out into the chest cavity making it hard to locate the met. It can be stabilised further by mixing it with autologous blood. It is also only useful for locating relatively superficial mets or as an adjunct to other localising techniques.
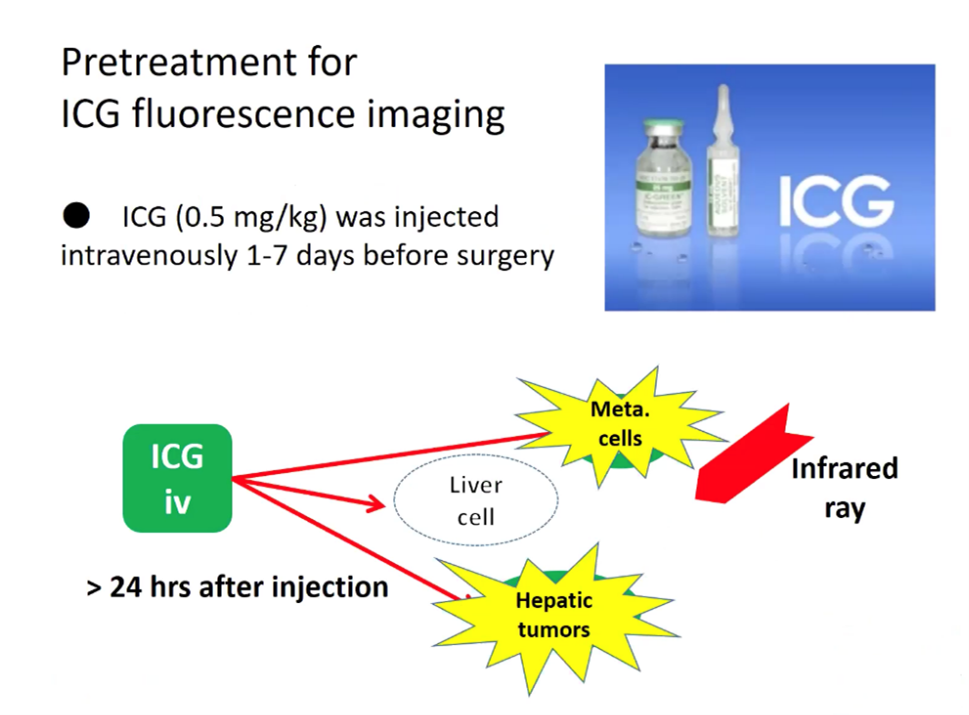
Indocyanine green (ICG)
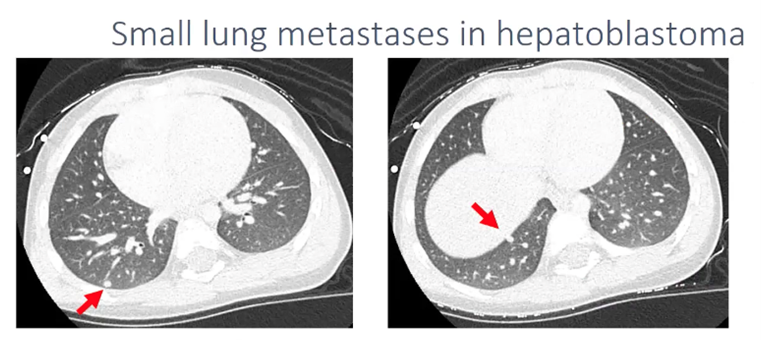
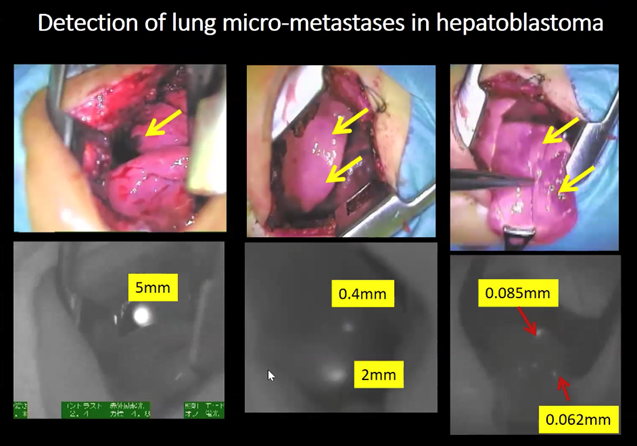
ICG fluorescent imaging (Fig. 6) allows real-time identification of cancer tissues. ICG was first discovered in 1976 and emits a light when illuminated with near-infrared light (NIR). ICG is taken up avidly by hepatocytes and can be used to assess liver function, it was discovered to be useful for liver tumour surgery by chance. It was noted to be retained longer in cancerous tissues as they were not connected with proper architecture to the biliary system. Since then ICG has been utilised for hepatectomies for adult hepatocellular carcinoma. Hepatoblastoma is the most common liver tumour in children and possesses similar features to adult HCC with ICG uptake and excretion. ICG can be injected at 0.5mg/kg 3-4 days prior to the surgery and this can enable the detection of lung micrometases using an infrared light at the time of thoracotomy (See figure 6 and 8). It can be more challenging with nodules that are not at the lung peripherally but compressing the lung gently to reduce the depth the metastasis is away from the surface can be of some assitance. Infrared cameras can now be built into most modern stack systems. It has also been used for other tumour types but with less reported success. This technique has the advantage that it is not invasive to the lung but the success depends on how well the tumour emits the light.
Lipiodol
CT guided lipiodol marking of PM has also been reported. Lipiodol is a poppyseed oil that can be used an injection directly into or surrounding the PM. It is a relatively cheap radio-opaque contrast agent that is held within the lung tissue. The marked nodule can be grasped under fluorscopy and then resected by means of thorascopy. Complications include air leak and bleeding and very rarely fat embolism has been reported.
Radiofrequency Ablation/Microwave Ablation (RFA/MWA)
RFA/MWA is uncommonly used in children with PM. Both techniques use accurate image guided insertion of needles into the PM to then produce a small region of heat around the PM with the aim of the heat destroying the cancer cells. It has the advantage that it does not require formal surgery and is a procedure entirely carried out by interventional radiology. This potentially allows the patient to make a faster recovery. The drawbacks are similar to all other invasive localisation techniques but it also has the added drawback of not gaining any histological diagnosis. It is not possible to characterise the lesion as a metasasis (viable or non viable), a granuloma or other cause (such as a fungal infection). It has been utilised as a technique for refractory/recurrent PM when the chance of cure is slim.
Conclusion
The hope with all these techniqes is better localisation of pulmonary metasases, the ability to define the true extent of the metasases and achieve lung parenchymal sparing surgery with a minimal complication rate. Technology is changing all the time and the exciting technique of hyperspectral imaging (HIS) is already in the pipeline which may further aid this field.
Further Reading
- Clinical Management Guidelines Renal Tumours. CCLG Renal Tumour Special Interest Group; January 2020. (Appendix 4)
- Complex table for lung metastases in Wilms tumour, but covers every eventuality. Published on CCLG Members’ area.
- Hwang S, Kim TG, Song YG. Comparison of hook wire versus coli localization for video-assisted thoracoscopic surgery. Thorac Cancer. 2018.; 9: 384-389.
- Two groups PM patients who underwent different thoracic localisation techniques were compared – Group A: hook wire – 45 patients, Group B: microcoil – 54 patients. The pneumothorax rate (~30%) and pulmonary parenchymal haemorrhage (~27%) was the same for both groups. There were 4 dislodgements in the hook wire group versus none in group B and the pain score was improved in the microcoil group. Both were significant.
- Buddingh EP, Anninga JK, Versteegh MI, et al. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer. 2010; 54: 216-21.
- This was a study following up 88 patients with osteosarcoma and pulmonary metastases either at diagnosis or relapse. 56 out of 88 patients underwent a surgical metastastectomy. The main prognostic factor was resectability of pulmonary metastases. Other factors associated with better outcomes included relapse free interval, female, fewer lung metastases and necrosis on histology. The survival for those children that had a pulmonary metastastectomy was only 23% at 120 months follow up.
- Whitlock RS, Patel KR, Yang T, et al. Pathologic correlation with near infrared-indocyanine green guided surgery for pediatric liver cancer. J Pediatr Surg. 2021; S0022-3468(21).
- 5 patients used intraoperative NIR-ICG guidance to aid PM resection using thoracoscopy or thoracotomy. Some of these lesions were not visible on conventional preoperative imaging however they were resected and found positive for malignancy.
- Watanabe K-Im Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computer tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopy resection of small pulmonary nodules: Experience with 174 nodules. J Thorac and Cardiovasc Surg. 2005, 132(2); 320-324.
- 174 patients (not children) – with PM ranging from 2-30mm (mean 10) had a preoperative injection of lipiodol. All the nodules could be marked however there was a range of complications with 11 patients requiring a chest drain from a pneumothorax.
- Zhang Y-T, Chang J, Yao Y-M, Li Y-N, Zhong X-D, Liu Z-L. Novel treatment of refractory / recurrent pulmonary hepatoblastoma. Pediatr Int. 2020; 62: 324-329.
- 12 children with refractory or recurrent PM (³2 lesions) underwent chemotherapy and RFA to their PM. 2 year EFS was 33% (4/12) and 2 year OS was 41.7% (5/12).
Curated by Mark Davenport (February 2022)
